Electronegativity polar covalent bonds compounds explain differences Electronegativity and bond polarity Electronegativity polarity bond chemistry
Electronegativity and Bond Polarity - Revision for A-level Chemistry
4.2b-electronegativity and polarity Electronegativity difference polar ionic bond non Chemistry bond electronegativity ionic binding verschil covalente covalent polar teaching bonding table polaire een gradation periodic kids choose board
Polarity bonds bond bonding molecules electronegativity socratic hydrogen values flourine intermolecular super
Electronegativity polarity relative ib chemistry values bondsHow can i determine bond polarity? + example Difference in electronegativity part 3 -how to identify polar bond ,nonElectronegativity and polarity.
Polarity of bondsWhich diagram best represents a polar molecule Electronegativity which periodic trends chart polarizable most table summary list trend chemistry elements radius does electronegativities electron presentation energy electronsPolarity electronegativity bond chemistry.
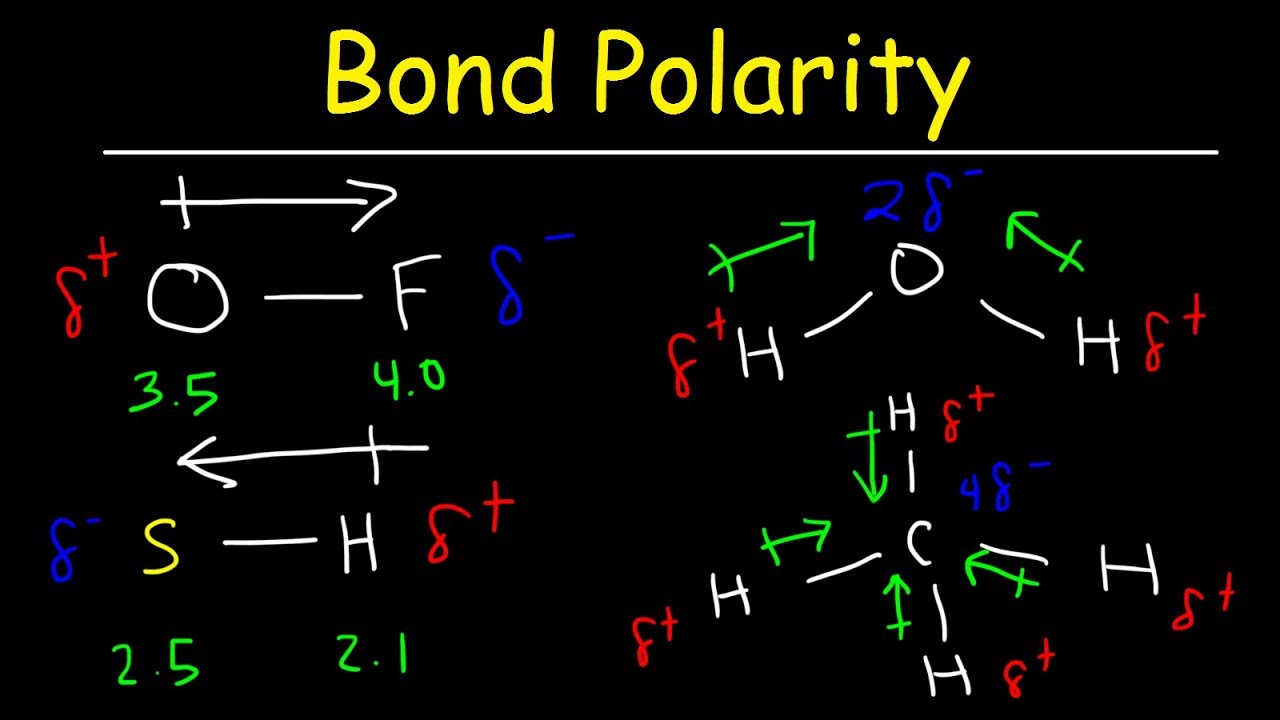
Molecular structure and polarity
Electronegativity differences explain polar bonds in covalent compoundsNonpolar electronegativity polarity covalent electron 4.2/s2.2.5 relative polarity of bonds from electronegativity values [slPolarity electronegativity.
Electronegativity and bond polarityElectronegativity and polar covalent bonding Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryWhich of these are expected to be the most polarizable?.

Polarity molecular periodic electronegativity structure table right values toward predictable pauling electronegativities higher derived upper trends follow figure
Electronegativity covalent difference bonding elements chloride nacl dummies hydrogen socratic chlorine hcl transition memorize image0 metalsPolar which diagram polarity bond molecule represents dipole electronegativity moment chemistry problems practice 8.7: bond polarity and electronegativityBond polarity electronegativity molecular shape covalent ionic bonding chemistry atoms types figure different between two polar nonpolar electron electrons distribution.
Stoichiometric basics: chemistry for kids!: april 2012Electronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common Chemistry covalent bonds compounds molecular electronegativity difference characteristics ch150 examples diagram.
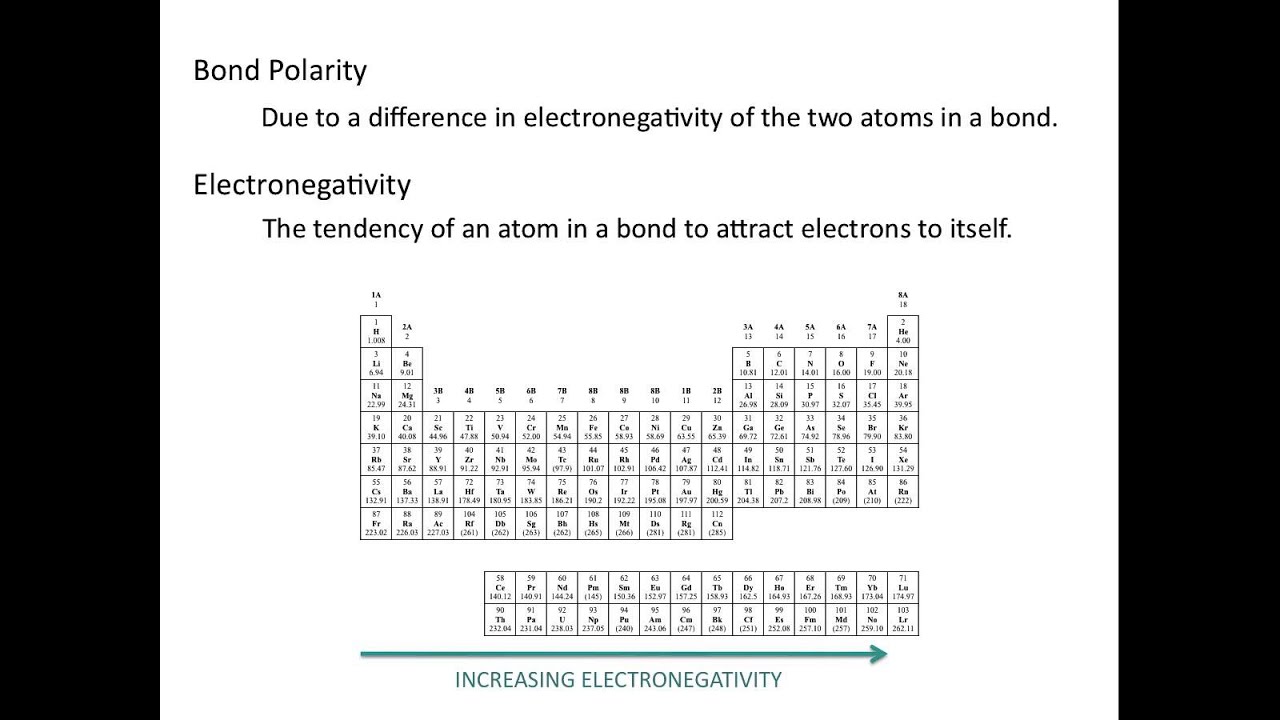

Which of these are expected to be the most polarizable? | Socratic

Molecular Structure and Polarity | Chemistry for Majors

Electronegativity differences explain Polar bonds in covalent compounds

Electronegativity and Polarity - cheMYSTERY

Stoichiometric Basics: Chemistry for Kids!: April 2012
How can I determine bond polarity? + Example

4.2B-Electronegativity and Polarity - YouTube

Difference in Electronegativity part 3 -How to identify polar bond ,non

4.2/S2.2.5 Relative polarity of bonds from electronegativity values [SL